Hard skills, which include technical competencies like staff management, budget oversight, and compliance with healthcare regulations, are essential for efficient operations.
Popular Supervisor Resume Examples
Check out our top supervisor resume examples that emphasize leadership skills, team management, and effective communication. These samples demonstrate how to showcase your achievements and make a positive impression on potential employers.
Ready to build your own impressive resume? Our Resume Builder offers user-friendly templates designed specifically for supervisors, helping you highlight your strengths with ease.
Recommended
Supervisor resume
The resume fonts chosen for the document's clean layout improve readability, making it easier for employers to quickly assess qualifications. These design elements not only highlight the applicant's skills and achievements but also demonstrate a commitment to professionalism that can leave a positive impression on hiring managers.
Team leader resume
This resume skillfully intertwines key abilities like team leadership and project management with substantial work experience. By presenting these skills alongside a proven track record of successful project delivery, employers can clearly assess the applicant's effectiveness in real-world scenarios and their potential contributions to future projects.
Assistant manager resume
This resume skillfully uses bullet points to clearly present a wealth of experience, making it easy for hiring managers to spot important achievements at a glance. The thoughtful layout, with ample spacing between sections, improves readability and ensures key qualifications stand out without cluttering the page.
Resume Template—Easy to Copy & Paste
Jin Lee
Riverside, CA 92507
(555)555-5555
Jin.Lee@example.com
Skills
- Team Leadership
- Operational Efficiency
- Project Management
- Workforce Training
- Inventory Management
- Process Improvement
- Safety Compliance
- Logistics Coordination
Certifications
- Certified Operations Manager - Operations Management Institute
- Process Improvement Specialist - Lean Six Sigma Group
Languages
- Spanish - Beginner (A1)
- French - Intermediate (B1)
- German - Beginner (A1)
Professional Summary
Experienced Supervisor specializing in operational excellence. Proven ability to lead teams, streamline processes, and enforce safety protocols, driving productivity by up to 30%. Expertise in logistics coordination and workforce training to ensure high-impact outcomes.
Work History
Supervisor
Summit Operations Group - Riverside, CA
January 2023 - November 2025
- Supervised a team of 15 employees, increasing productivity by 20%.
- Implemented safety protocols, reducing workplace incidents by 10%.
- Streamlined operational workflows, cutting delays by 30%.
Operations Coordinator
ProVista Logistics - Riverside, CA
January 2020 - December 2022
- Coordinated logistics for 50+ supply chain projects annually.
- Reduced inventory discrepancies by 25% through process audits.
- Trained 12 new hires, achieving 95% retention rate.
Team Lead
Vista Industrial Solutions - San Francisco, CA
January 2017 - December 2019
- Led cross-functional teams to complete 0K in annual projects.
- Improved order fulfillment rate by 15% via optimized scheduling.
- Introduced new training modules, enhancing efficiency by 10%.
Education
Master's Degree Operations Management
University of California, Berkeley Berkeley, California
May 2017
Bachelor's Degree Business Administration
California State University Long Beach, California
May 2015
How to Write a Supervisor Resume Summary
Your resume summary is the first thing employers will see, making it important to create a strong impression. This section should showcase your unique leadership skills and relevant experience that align with the supervisor role.
As a supervisor, you need to highlight your abilities in team management, communication, and problem-solving. These qualities demonstrate your capacity to lead effectively and drive results.
To help you grasp what makes an strong resume summary, consider these examples of successful summaries tailored for supervisors. They will illustrate key components that resonate with hiring managers:
I am an experienced supervisor seeking a new opportunity where I can use my knowledge and contribute to a successful team. I believe that a supportive work environment with chances for advancement is essential for my growth. I am eager to collaborate and ensure productivity in the workplace if given a chance.
- Vague phrases like 'experienced' and 'knowledge' do not clearly define skills or accomplishments
- Overuse of personal language detracts from professionalism and substance
- Emphasizes what the job seeker desires rather than outlining specific value they bring to the employer
Results-driven supervisor with over 7 years of experience in managing diverse teams and improving operational efficiency. Achieved a 20% increase in team productivity by implementing streamlined processes and performance metrics. Proficient in project management, conflict resolution, and employee training programs to improve staff development and customer satisfaction.
- Begins with clear years of experience and role focus
- Highlights measurable achievements that reflect an impact on organizational performance
- Showcases relevant skills that align with supervisory responsibilities
Pro Tip
Showcasing Your Work Experience
The work experience section is important for your resume as a supervisor, where you'll find the bulk of your content. Good resume templates always emphasize this key area to highlight your professional journey.
This section should be organized in reverse-chronological order, listing your previous roles clearly. Use bullet points to detail your achievements and responsibilities in each position, making it easy for hiring managers to see your impact.
Now, let’s look at a couple of examples that showcase effective work history entries for supervisors. These examples will illustrate what stands out and what might miss the mark:
Supervisor
ABC Manufacturing – Dallas, TX
- Oversaw staff operations
- Managed schedules and shifts
- Handled reports and documentation
- Addressed employee concerns as they arose
- Lacks specific details about the responsibilities held
- Bullet points do not highlight achievements or leadership skills
- Vague descriptions do not demonstrate impact on team performance
Supervisor
Tech Innovations Inc. – San Francisco, CA
March 2020 - Current
- Lead a team of 15 in the development and execution of software solutions, achieving a 30% increase in project delivery speed
- Implement performance metrics that improved team productivity by 20%, fostering a culture of accountability and excellence
- Conduct training sessions for staff on emerging technologies, resulting in a 40% improvement in skill skill within six months
- Uses compelling action verbs to clearly convey the applicant’s leadership contributions
- Incorporates specific percentages to highlight measurable improvements in performance and efficiency
- Demonstrates relevant supervisory skills using detailed examples of team management and training
While the resume summary and work experience are important components of your resume, don't overlook the importance of other sections. Each part plays a significant role in presenting your qualifications effectively. For further insights, visit our complete guide on how to write a resume.
Top Skills to Include on Your Resume
A well-defined skills section is important for any effective resume. It allows potential employers to see at a glance that you possess the necessary qualifications and competencies for the supervisor role.
For supervisors, emphasize both technical skills and interpersonal abilities. Highlight proficiency with project management software, familiarity with performance tracking systems, and strong communication skills to enhance team collaboration and productivity.
Equally important are soft skills, such as leadership, conflict resolution, and effective communication. These abilities play a important role in fostering a collaborative environment and ensuring high-quality patient care. For more on this topic, explore the importance of hard skills in various job roles.
Selecting the right resume skills is important for aligning with what employers expect in an applicant. Many companies use automated systems to filter out applicants who lack essential skills for the position.
To ensure your resume stands out, carefully review job postings for insights on which skills to highlight. This approach not only appeals to recruiters but also improves your chances of passing through ATS filters effectively.
Pro Tip
10 skills that appear on successful supervisor resumes
Highlighting essential skills on your resume can significantly capture the attention of recruiters seeking supervisors. By exploring our resume examples, you’ll discover these and other key qualifications, empowering you to apply with confidence.
Here are 10 vital skills you might want to include in your resume if they resonate with your experience and job expectations:
Customer service
Decision-making
Team building
Training and mentoring
Attention to detail
Schedule development
Goal oriented
Inventory control
Relationship building
Operations management
Based on analysis of 5,000+ management professional resumes from 2023-2024
Resume Format Examples
Choosing the right resume format is important because it highlights your leadership abilities, relevant experience, and career advancements, creating a strong impression on potential employers.
Functional
Focuses on skills rather than previous jobs
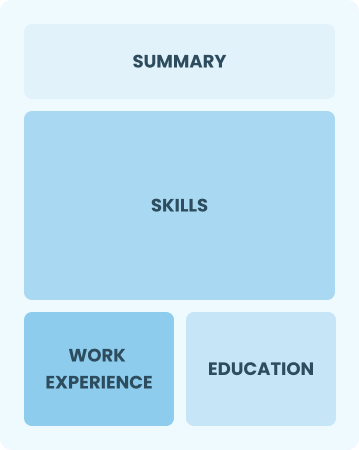
Best for:
Recent graduates and career changers with limited experience in the field
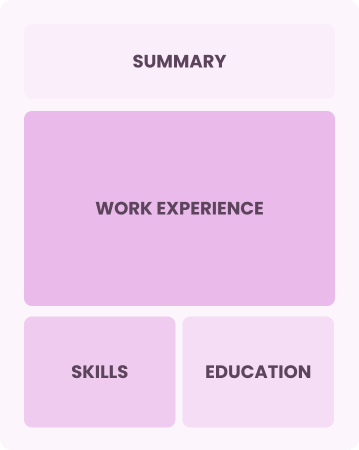
Combination
Balances skills and work history equally
Best for:
Mid-career professionals focused on demonstrating their skills and potential for growth
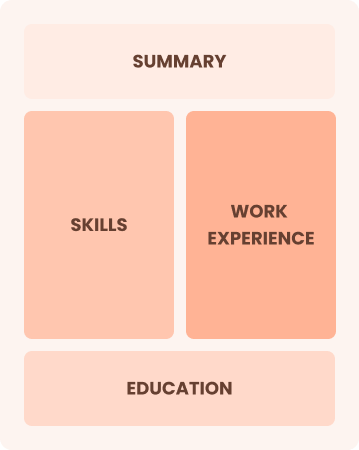
Chronological
Emphasizes work history in reverse order
Best for:
Experienced leaders driving innovation in healthcare practices
Supervisor Salaries in the Highest-Paid States
Our supervisor salary data is based on figures from the U.S. Bureau of Labor Statistics (BLS), the authoritative source for employment trends and wage information nationwide.
Whether you're entering the workforce or considering a move to a new city or state, this data can help you gauge what fair compensation looks like for supervisors in your desired area.
Frequently Asked Questions
Should I include a cover letter with my supervisor resume?
Absolutely, including a cover letter can significantly improve your application. It gives you the opportunity to personalize your submission and highlight key experiences related to the position. If you're looking for tips on how to write a cover letter, consider checking our guide or using our Cover Letter Generator for quick assistance.
Can I use a resume if I’m applying internationally, or do I need a CV?
When applying for jobs outside the U.S., use a CV instead of a resume to provide a comprehensive overview of your academic and professional history. For guidance on formatting and creating an effective CV, explore our resources that offer CV examples and tips tailored for international applications. If you're unsure how to write a CV, these resources will assist in crafting one that stands out.
What soft skills are important for supervisors?
Soft skills like leadership, conflict resolution, and active listening are essential for supervisors. These interpersonal skills help create a positive work environment, improve team collaboration, and support effective communication, which contributes to better performance and increased employee satisfaction.
I’m transitioning from another field. How should I highlight my experience?
When applying for supervisor roles, make sure to highlight your transferable skills such as communication, teamwork, and adaptability. These abilities showcase your value even if your experience in the specific field is limited. Share concrete examples from past positions that illustrate how you've successfully managed teams or projects to align with the responsibilities of a supervisor.
Where can I find inspiration for writing my cover letter as a supervisor?
If you're aiming for a supervisor position, consider exploring our collection of professionally crafted cover letter examples. These resources can spark content ideas, provide formatting tips, and help you articulate your qualifications in a compelling way. With the right inspiration, your application will stand out.
How do I write a resume with no experience?
If you're applying for a supervisor position with limited experience, focus on showcasing your leadership capabilities through group projects, volunteer roles, or relevant coursework. For guidance on crafting a resume with no experience, highlight skills such as problem-solving, communication, and teamwork. Remember, employers value potential and a positive attitude just as much as past job titles. Your unique experiences can truly set you apart.







