Hard skills are essential technical abilities such as strategic planning, financial management, and regulatory compliance that a vice president must demonstrate to drive organizational success.
Popular Vice President Resume Examples
Discover our top vice president resume examples that emphasize leadership skills, strategic planning, and business development achievements. These examples will guide you in showcasing your qualifications effectively.
Ready to design your ideal resume? Our Resume Builder offers user-friendly templates specifically crafted for executives, making it easier than ever to highlight your experience.
Recommended
Vice president resume
The resume's clean layout and professional resume fonts choices improve readability, allowing the applicant's accomplishments to stand out clearly. This strategic use of design elements effectively shows their qualifications and achievements, making a compelling impression on potential employers.
Senior vice president resume
This resume effectively combines key skills such as strategic planning and financial analysis with extensive work experience. By illustrating these abilities alongside real achievements, employers can better grasp the applicant's potential impact on their organization, showcasing both expertise and proven results in leadership roles.
Executive vice president resume
This resume showcases extensive experience through clear bullet points that succinctly detail key achievements, making it easy for hiring managers to identify qualifications at a glance. The use of strategic spacing and distinct sections ensures important information stands out while maintaining an organized and professional layout.
Resume Template—Easy to Copy & Paste
Jane Williams
Dallas, TX 75201
(555)555-5555
Jane.Williams@example.com
Skills
- Strategic Planning
- Team Leadership
- Operational Efficiency
- Budget Management
- Process Improvement
- Customer Satisfaction
- Project Management
- Change Management
Languages
- Spanish - Beginner (A1)
- German - Beginner (A1)
- French - Beginner (A1)
Professional Summary
Seasoned executive with expertise in strategic planning and operations. Proven track record of driving growth and maximizing efficiency, enhancing customer satisfaction by implementing innovative solutions.
Work History
Vice President
Apex Technologies - Dallas, TX
January 2024 - October 2025
- Implemented new strategies, increased revenue by 15%
- Led a team of 50+, improving productivity by 30%
- Enhanced customer satisfaction by 20% in the first year
Director of Operations
Innovative Solutions Inc. - Pinehill, TX
January 2022 - December 2023
- Streamlined processes, reducing costs by 25%
- Managed annual budgets over 10 million
- Boosted team efficiency, completing projects 10% faster
Operations Manager
Pinnacle Enterprises - Austin, TX
January 2020 - December 2021
- Reduced inventory loss by 30% through new protocols
- Improved supplier relations, cutting lead times by 15%
- Increased operational efficiency leading to 20% saving
Certifications
- Certified Management Professional - Institute of Management
- Senior Project Manager - Project Management Institute
Education
Master of Business Administration Business Administration
Harvard University Cambridge, MA
June 2020
Bachelor of Science Management
University of California, Berkeley Berkeley, CA
June 2018
How to Write a Vice President Resume Summary
Your resume summary is the initial hook that can captivate employers' attention. It’s essential to effectively convey your leadership experience and strategic vision in this key section.
As a vice president, you should emphasize your ability to drive organizational growth, inspire teams, and implement successful initiatives. Highlighting these attributes will set you apart in a competitive job market.
To illustrate what makes an strong summary, we’ll provide examples that demonstrate effective strategies and common pitfalls:
I am an experienced vice president with a successful career in leadership. I want to find a company where I can use my skills and be part of a great team. A position that allows for personal growth and good benefits is important to me. I believe I could add value if given a chance.
- Lacks specific examples of leadership achievements or skills relevant to the vice president role
- Overuses personal pronouns and vague phrases, making it sound generic rather than compelling
- Emphasizes what the job seeker seeks instead of detailing how their experience benefits potential employers
Strategic vice president with over 10 years of experience in driving organizational growth through innovative business strategies and operational excellence. Achieved a 25% increase in annual revenue by implementing data-driven decision-making processes and improving customer engagement initiatives. Proficient in cross-functional leadership, market analysis, and developing sustainable business models that align with corporate vision.
- Begins with clear experience level and specific role focus
- Highlights quantifiable achievements that show impact on company performance
- Showcases essential skills relevant to strategic leadership roles
Pro Tip
Showcasing Your Work Experience
The work experience section is important for your resume as a vice president, serving as the primary focus of your content. Good resume templates always emphasize this area to highlight your leadership roles and accomplishments.
This section should be organized in reverse-chronological order, detailing your previous positions. Use bullet points to outline key achievements and contributions that demonstrate your impact in each role.
To illustrate effective entries for a vice president's work history, we’ve prepared a couple of examples. These will clarify what stands out in a resume and what pitfalls to avoid.
Vice President
Global Enterprises – New York, NY
- Managed teams and projects
- Conducted meetings and collaborated with others
- Oversaw company operations
- Handled budgets and reports
- Lacks specific employment dates for context
- Bullet points are overly generic and do not highlight leadership achievements
- Focuses on basic responsibilities rather than powerful results or innovations
Vice President
Tech Innovations Inc. – San Francisco, CA
March 2020 - Current
- Lead strategic initiatives that increased annual revenue by 40%, improving market competitiveness and operational efficiency
- Foster partnerships with key stakeholders, resulting in a 30% growth in client retention over three years
- Mentor and develop executive team members, promoting leadership skills that improved overall team performance metrics by 25%
- Starts each bullet point with engaging action verbs that highlight the job seeker’s achievements
- Incorporates specific percentages to quantify success and impact on the organization
- Demonstrates relevant skills such as leadership and strategic planning critical for a vice president role
While the resume summary and work experience sections are important, don't overlook other areas that also need your attention. For detailed guidance on crafting a well-rounded resume, be sure to explore our comprehensive guide on how to write a resume.
Top Skills to Include on Your Resume
A well-defined skills section is important for any strong resume. It allows you to clearly demonstrate your qualifications and helps employers quickly identify your potential fit for the role.
For a vice president, showcasing hard skills and soft skills equally on a resume is crucial to landing a job interview. While hard skills are indispensable for a VP, soft skills are those interpersonal abilities that make you stand out and help you become an effective leader. Both skill types are equally important for anyone applying to a vice president role.
Soft skills, including leadership, communication, and problem-solving abilities, play an important role in fostering collaboration and motivating teams to achieve common goals.
Choosing the appropriate resume skills is essential to align with employer expectations and navigate automated screening processes. These systems often look for specific qualifications, so including relevant skills can significantly boost your chances.
Thoroughly reviewing job listings helps determine which skills to highlight. They offer valuable insights into what recruiters and ATS systems prioritize, ensuring your resume stands out effectively.
Pro Tip
10 skills that appear on successful vice president resumes
Highlighting key skills on your resume can significantly increase your appeal to recruiters searching for vice president job seekers. These essential skills are frequently showcased in resume examples, allowing you to apply with the self-assurance that a well-crafted resume offers.
By the way, consider integrating these valuable skills into your resume when they align with your expertise and job specifications:
Strategic planning
Leadership
Financial acumen
Market analysis
Project management
Negotiation skills
Team development
Data-driven decision making
Stakeholder engagement
Operational efficiency
Based on analysis of 5,000+ administrative professional resumes from 2023-2024
Resume Format Examples
Selecting the right resume format for a vice president role is important to showcase your leadership skills, strategic accomplishments, and executive career growth effectively.
Functional
Focuses on skills rather than previous jobs
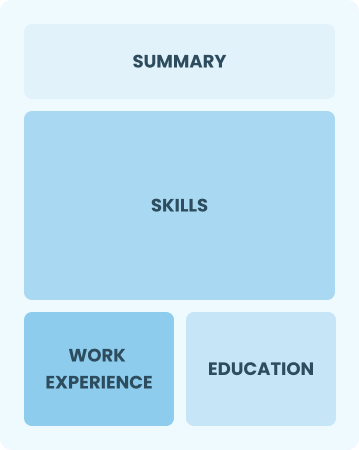
Best for:
Recent graduates and career changers with limited experience
Combination
Balances skills and work history equally
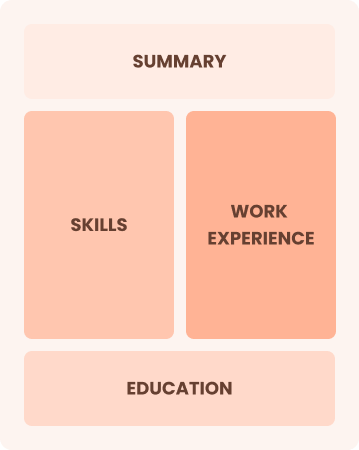
Best for:
Mid-career professionals focused on demonstrating their skills and potential for growth
Chronological
Emphasizes work history in reverse order
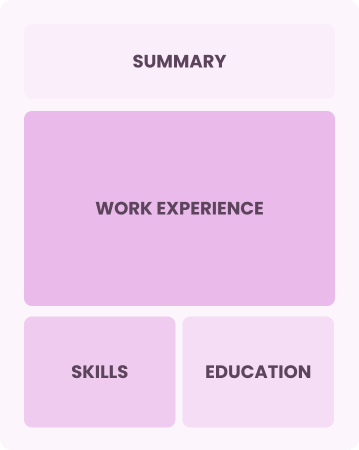
Best for:
Leaders driving innovation and strategy in management
Frequently Asked Questions
Should I include a cover letter with my vice president resume?
Absolutely. A cover letter is a valuable tool in your job application, allowing you to showcase your personality and passion for the role. It can help differentiate you from other job seekers by highlighting specific experiences that align with the job. If you need assistance, explore our comprehensive guide on how to write a cover letter or use our Cover Letter Generator for quick support.
Can I use a resume if I’m applying internationally, or do I need a CV?
When applying for jobs abroad, use a CV instead of a resume. A CV is often preferred in many countries as it provides a comprehensive view of your professional history. Explore our resources on how to write a CV and check out these CV examples to ensure your application stands out internationally.
What soft skills are important for vice presidents?
Soft skills like leadership, emotional intelligence, and effective communication are essential for vice presidents. These interpersonal skills foster strong relationships within teams and promote collaboration across departments, ultimately driving organizational success and a positive workplace culture.
I’m transitioning from another field. How should I highlight my experience?
Highlight skills like strategic thinking, team leadership, and project management from your previous roles. These abilities showcase your transferable skills and potential to thrive in a vice president position, even if you lack direct experience in the sector. Use concrete examples to illustrate how your past successes align with the responsibilities of this executive role.
Should I use a cover letter template?
Using a cover letter template is advisable because it provides a clear structure that organizes your clinical skills, leadership experience, and patient care achievements effectively. This approach makes it easier for hiring managers to identify your qualifications and fit for the vice president role.
How do I write a resume with no experience?
If you're aiming for a vice president position but lack extensive work experience, consider using a resume with no experience to highlight your leadership roles in projects, relevant coursework, and any volunteer initiatives. Showcase transferable skills like strategic thinking and team collaboration. Employers value potential and dedication, so focus on how your unique experiences can contribute to their goals.







