Mastery of hard skills is essential for product managers, encompassing technical competencies such as market analysis, product lifecycle management, and data-driven decision-making.
Popular Product Manager Resume Examples
Check out our top product manager resume examples that emphasize key skills such as strategic planning, market analysis, and cross-functional collaboration. By reviewing these examples, you can effectively showcase your experience and achievements to potential employers.
If you're ready to build an impressive resume, take advantage of our Resume Builder. It offers user-friendly templates specifically designed for product management professionals, helping your job application stand out.
Recommended
Product manager resume
This resume features organized sections and a modern resume font that improve readability, allowing hiring managers to quickly identify key qualifications. The clean layout not only reflects professionalism but also emphasizes the job seeker's achievements, making a positive impression on potential employers.
Senior product manager resume
This resume effectively combines key skills such as agile project management and user experience improvement with substantial work experience. By presenting these strengths alongside a proven track record in revenue growth and team leadership, employers can clearly see how this applicant's capabilities align with their professional achievements.
Product marketing manager resume
This resume uses clear headings and bullet points to effectively showcase a wealth of experience in product marketing. The strategic spacing between sections allows hiring managers to quickly identify the job seeker's key achievements and skills, ensuring important information is easily accessible at a glance.
Resume Template—Easy to Copy & Paste
Li Smith
Brookfield, WI 53008
(555)555-5555
Li.Smith@example.com
Professional Summary
Accomplished Product Manager with 8 years of experience leading projects, launching products, and driving growth. Expert in agile methodologies, data analysis, and team leadership.
Work History
Product Manager
Innovatech Solutions - Brookfield, WI
January 2022 - November 2025
- Led a team to increase product adoption by 40%
- Launched three successful products generating M revenue
- Implemented agile methodologies improving delivery by 25%
Product Owner
NextGen Softwares - Brookfield, WI
January 2018 - December 2021
- Oversaw product roadmap leading to a 30% user growth
- Coordinated with cross-functional teams increasing efficiency by 20%
- Introduced data-driven decision making boosting profitability
Project Coordinator
TechFusion Corp - Milwaukee, WI
January 2017 - December 2017
- Managed a budget of 1M ensuring project success
- Facilitated team meetings improving output by 15%
- Directed project timelines reducing delays by 10%
Skills
- Product Lifecycle Management
- Agile Methodologies
- Market Research
- Data Analysis
- Team Leadership
- Strategic Planning
- Cross-functional Collaboration
- User Experience Design
Certifications
- Certified Scrum Product Owner (CSPO) - Scrum Alliance
- Project Management Professional (PMP) - Project Management Institute
Education
Master of Business Administration Business Management
Harvard University Cambridge, MA
May 2017
Bachelor of Science Computer Science
University of California, Berkeley Berkeley, CA
May 2015
Languages
- Spanish - Beginner (A1)
- French - Intermediate (B1)
- German - Beginner (A1)
How to Write a Product Manager Resume Summary
Your resume summary is the first opportunity to grab an employer's attention, making it important to present your qualifications effectively. As a product manager, you need to showcase your leadership in project management and ability to drive results through innovation and collaboration. To illustrate what makes an effective summary for this role, we'll look at examples that highlight strong points and areas to avoid:
I am a dedicated product manager with extensive experience in the field and a passion for creating successful products. I am seeking a position where I can apply my skills and drive growth for the company. A team-focused environment that values innovation and collaboration is what I’m looking for. I believe my background will be beneficial to your organization.
- Uses vague phrases like 'extensive experience' without providing specific achievements or metrics
- Emphasizes personal desires over contributions, failing to highlight how the applicant can add value to the employer
- Contains generic language that doesn't distinguish the job seeker from others in similar roles
Results-driven product manager with over 7 years of experience in leading cross-functional teams to launch innovative software solutions. Achieved a 30% increase in customer retention through the implementation of data-driven user feedback processes and agile methodologies. Proficient in product lifecycle management, market analysis, and stakeholder engagement to ensure alignment with business objectives.
- Begins with clear years of experience and specific role focus
- Highlights quantifiable achievements that illustrate impact on customer retention
- Details relevant skills that demonstrate skill in key product management areas
Pro Tip
Showcasing Your Work Experience
The work experience section is the backbone of your resume as a product manager, where you’ll present the majority of your content. A well-crafted resume always emphasizes this important area with resume templates.
Organize this section in reverse-chronological order and use bullet points to highlight your key achievements and responsibilities in each role you've held.
To further illustrate the importance of an effective work history for product managers, we will showcase examples that demonstrate what captures attention and what doesn’t work as well.
Product Manager
Tech Innovations Inc. – San Francisco, CA
- Managed product development.
- Collaborated with teams.
- Conducted market research.
- Oversaw project timelines.
- Lacks specific achievements or results from projects managed
- Bullet points are overly generic and do not showcase unique skills
- No mention of key metrics that demonstrate impact on business outcomes
Product Manager
Tech Innovations Inc. – San Francisco, CA
March 2020 - Present
- Lead cross-functional teams to launch five successful products, achieving a 40% increase in market share within one year.
- Conduct market research and user testing, driving product improvements that resulted in a 30% boost in customer satisfaction scores.
- Develop and manage product roadmaps, ensuring alignment with strategic goals and timely delivery of all project milestones.
- Starts each bullet with compelling action verbs that highlight the job seeker's contributions
- Incorporates specific metrics to quantify achievements and impact on the business
- Demonstrates relevant skills by showcasing leadership, analytical thinking, and strategic planning
While your resume summary and work experience are important, don’t overlook the importance of other sections that also deserve careful attention. For detailed guidance on crafting a well-rounded resume, be sure to explore our comprehensive guide on how to write a resume.
Top Skills to Include on Your Resume
A well-defined skills section is important for any resume, as it allows you to showcase your qualifications quickly. This section helps hiring managers see at a glance that you possess the abilities needed for the product manager role.
A mix of hard and soft skills helps your application stand out.
Equally important are soft skills, which include interpersonal attributes like leadership, adaptability, and effective communication. These skills foster collaboration among teams and ensure the successful delivery of products that meet customer needs.
Selecting the right resume skills is important for aligning with employer expectations and overcoming automated screening systems. Many companies rely on software to filter out applicants lacking essential skills, so it's important to showcase your qualifications effectively.
To improve your chances, carefully review job postings for insights into which skills are most relevant. By tailoring your resume to highlight these key competencies, you can better attract recruiter attention and ensure compatibility with ATS requirements.
Pro Tip
7 skills that appear on successful product manager resumes
Improve your resume to attract recruiters by highlighting key skills that are in high demand for product managers. These abilities are demonstrated in resume examples, helping you feel confident applying to positions with a polished presentation.
Here are 7 essential skills you should consider including in your resume if they align with your experiences and job specifications:
Product vision and strategy
End to end feature delivery
Timeline management
Strategic planning
Stakeholder management
Teamwork and collaboration
Business development
Based on analysis of 5,000+ management professional resumes from 2023-2024
Resume Format Examples
Selecting the appropriate resume format is important for product managers as it emphasizes essential skills, relevant experiences, and career growth in a clear and engaging manner.
Functional
Focuses on skills rather than previous jobs
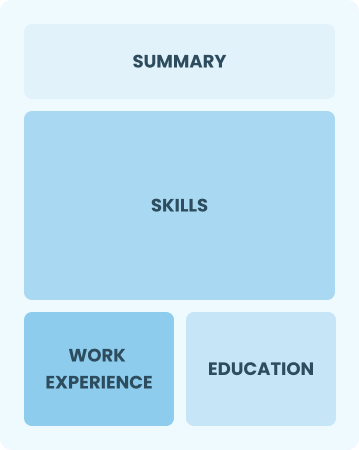
Best for:
Recent graduates and career changers with up to two years of experience
Combination
Balances skills and work history equally
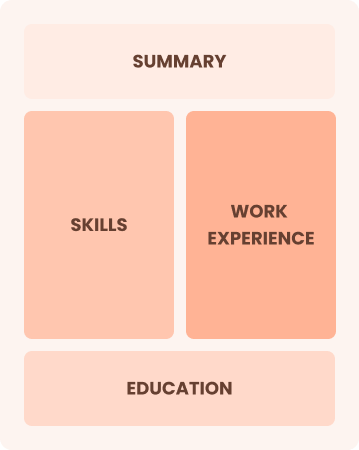
Best for:
Mid-career professionals seeking to highlight skills and leadership growth
Chronological
Emphasizes work history in reverse order
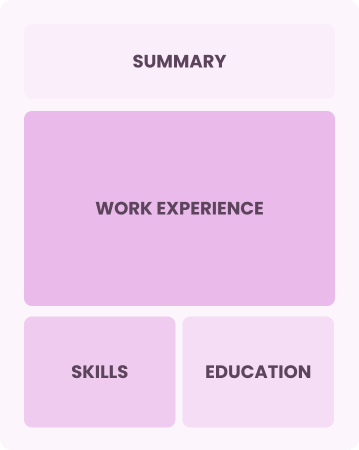
Best for:
Seasoned leaders in innovative healthcare management
Frequently Asked Questions
Should I include a cover letter with my product manager resume?
Absolutely, including a cover letter can significantly improve your application. It allows you to showcase your personality and articulate your passion for the role. If you're unsure about crafting one, we provide helpful resources such as guidance on how to write a cover letter and an easy-to-use Cover Letter Generator that can assist you in creating a compelling letter quickly.
Can I use a resume if I’m applying internationally, or do I need a CV?
When applying for jobs abroad, use a CV instead of a resume if the employer requests it or if you're targeting positions in regions where CVs are standard. Learn how to write a CV and explore CV examples to ensure your application stands out globally.
What soft skills are important for product managers?
Soft skills like communication, collaboration, and adaptability are essential for product managers. These interpersonal skills foster strong relationships within teams and with stakeholders, facilitating smoother project execution and driving successful product outcomes.
I’m transitioning from another field. How should I highlight my experience?
When applying for product manager roles, highlight your transferable skills such as communication, strategic thinking, and project management. These abilities demonstrate your potential impact on product development, even if you lack direct experience. Use concrete examples from previous jobs to illustrate how your achievements align with key responsibilities in product management.
How should I format a cover letter for a product manager job?
When you format a cover letter, start with your contact information and a professional salutation. Next, include an engaging introduction that captures attention, followed by detailing your relevant qualifications and experiences. Align your content with the specific job requirements to demonstrate you’re an ideal fit. Finish with a strong closing statement that encourages further discussion.
Should I use a cover letter template?
Yes, using a cover letter template is advisable for product managers as it ensures a clear structure that highlights essential skills such as market analysis and project management. A tailored approach with the right template helps organize your achievements effectively, making it easier for hiring managers to recognize your qualifications at a glance.







