Hard skills include project planning, budgeting, resource allocation, and risk management.
Popular Project Manager Resume Examples
Check out our top project manager resume examples that emphasize important skills such as strategic planning, risk management, and team leadership. These examples will help you effectively showcase your accomplishments to potential employers.
Ready to build your impressive resume? Our Resume Builder offers user-friendly templates specifically designed for project management professionals, making the process straightforward and efficient.
Recommended
Entry-level project manager resume
This entry-level resume for a project manager effectively highlights the job seeker's strong leadership abilities and successful management of projects within budget constraints, showcasing a solid foundation in agile methodologies. New professionals in this field must convey their capacity for strategic planning and stakeholder engagement to demonstrate their readiness to deliver results, even with limited direct experience.
Mid-career project manager resume
This resume effectively showcases key qualifications by highlighting leadership roles and measurable achievements. The structured presentation of experiences illustrates a clear career progression, demonstrating readiness to tackle complex projects and drive strategic initiatives.
Experienced project manager resume
The work experience section emphasizes the applicant's exceptional project management skills, notably increasing team efficiency by 20% and completing projects ahead of schedule. The use of bullet points aids in quick readability for hiring managers.
Resume Template—Easy to Copy & Paste
Aya Johnson
Louisville, KY 40205
(555)555-5555
Aya.Johnson@example.com
Skills
- Project Management
- Agile Methodologies
- Budget Management
- Team Leadership
- Process Improvement
- Data Analysis
- Communication Skills
- Vendor Management
Certifications
- PMP Certification - Project Management Institute
- Certified ScrumMaster - Scrum Alliance
Languages
- Spanish - Beginner (A1)
- French - Bilingual or Proficient (C2)
- German - Intermediate (B1)
Professional Summary
Dynamic Project Manager with 8 years of experience in team leadership, agile methodologies, and budget management. Proven success in optimizing workflows and delivering projects under budget and on time.
Work History
Project Manager
Tech Innovators Inc. - Louisville, KY
January 2023 - November 2025
- Led project team of 15, achieving 20% under budget.
- Implemented Agile processes, increasing efficiency by 30%.
- Managed 2M project portfolio, delivered on time.
Senior Project Coordinator
Future Tech Solutions - Louisville, KY
January 2019 - December 2022
- Coordinated team efforts, boosting productivity by 25%.
- Oversaw budget of 1.5M for key projects.
- Streamlined communication, reducing delays by 15%.
Project Analyst
Innovation Hub - Louisville, KY
January 2017 - December 2018
- Analyzed project data, improving metrics by 10%.
- Supported project planning, enhancing timelines by 20%.
- Managed databases, reducing errors by 15%.
Education
Master of Business Administration Business Management
Stanford University Stanford, CA
June 2017
Bachelor of Science Industrial Engineering
University of California, Berkeley Berkeley, CA
June 2015
How to Write a Project Manager Resume Summary
Your resume summary is the first thing employers see, making it important to create a lasting impression that highlights your qualifications. As a project manager, you should emphasize your leadership skills, successful project completions, and ability to meet deadlines while managing teams.
This profession requires showcasing not just your technical abilities but also your strategic thinking and communication skills. You want to demonstrate how you've driven projects forward and facilitated collaboration among stakeholders.
To help illustrate what makes an effective resume summary for a project manager, here are some examples that will show you what works well and what doesn’t:
I am an experienced project manager who has worked on many different projects. I want to find a job that allows me to use my skills and grow in my career. A company with good team dynamics and opportunities for advancement would be great. I believe I can contribute effectively if given the chance.
- Lacks specific details about the job seeker's achievements or expertise in project management
- Relies heavily on personal desires rather than highlighting what value the applicant brings to potential employers
- Uses generic phrases that fail to convey unique qualifications or strengths relevant to project management
Results-driven project manager with over 6 years of experience leading cross-functional teams in delivering complex projects on time and within budget. Successfully managed project budgets averaging $1M, achieving a 20% reduction in costs through strategic resource allocation and process optimization. Proficient in Agile methodologies, risk management, and stakeholder communication, ensuring alignment with organizational goals.
- Starts with a clear statement of experience level and context of work
- Highlights quantifiable achievements that showcase both budget management and cost savings
- Mentions specific technical skills relevant to project management, improving credibility
Pro Tip
Showcasing Your Work Experience
The work experience section is the cornerstone of your resume as a project manager, where you will include the bulk of your content. Good resume templates always feature this important section prominently.
This part should be organized in reverse-chronological order, listing your previous positions. Use bullet points to detail your achievements and the impact you made in each role.
To help illustrate effective strategies for presenting your work history, we’ve prepared a couple of examples that show what works well and what doesn’t:
Project Manager
XYZ Solutions – New York, NY
- Managed various projects.
- Coordinated with team members.
- Communicated with clients about progress.
- Ensured deadlines were met.
- Lacks specific employment dates
- Vague bullet points do not highlight key achievements or skills
- Emphasis on basic responsibilities rather than measurable successes
Project Manager
Tech Innovations LLC – San Francisco, CA
March 2020 - Present
- Lead cross-functional teams in the successful launch of five major software products, achieving a 30% increase in customer satisfaction ratings.
- Streamline project workflows by implementing Agile methodologies, resulting in a 40% reduction in project delivery times.
- Facilitate regular stakeholder meetings to ensure alignment on project goals and timelines, fostering transparent communication across departments.
- Starts each bullet with action verbs that clarify the applicant's contributions
- Incorporates specific metrics to illustrate tangible achievements and improvements
- Highlights essential skills relevant to project management while showcasing leadership capabilities
While your resume summary and work experience are important components, don't overlook the importance of other sections that can improve your overall presentation. For more detailed insights, be sure to explore our complete guide on how to write a resume.
Top Skills to Include on Your Resume
A skills section is important for any standout resume because it helps employers quickly evaluate whether you have the technical skills needed to succeed in the role.
As a project manager, focus on showcasing your organizational and leadership abilities. Include technical skills such as experience with project management software like Microsoft Project or Jira, which are critical for managing timelines and fostering team collaboration effectively.
These skills are essential for successfully overseeing projects from initiation to completion. Soft skills such as leadership, communication, and problem-solving abilities foster collaboration among team members and ensure successful project outcomes.
When selecting resume skills for your resume, it's important to align them with what employers expect. This approach helps you stand out, especially since many companies use automated systems to filter resumes lacking essential skills.
To make sure your resume resonates with recruiters and passes ATS screening, carefully examine job postings. They often offer valuable insights into which specific skills should be emphasized to attract attention.
Pro Tip
10 skills that appear on successful project manager resumes
Make your resume stand out to recruiters by highlighting the essential skills that project managers possess. Our resume examples illustrate these qualifications, allowing you to approach job applications with increased confidence.
By the way, consider incorporating any relevant skills from the list below that align with your experience and job specifications:
Strategic planning
Budget control
Project management
Project tracking
Cross-functional collaboration
Team collaboration
Risk management
Agile methodology
Project planning
Data analysis
Based on analysis of 5,000+ management professional resumes from 2023-2024
Resume Format Examples
Choosing the best resume format is important for a project manager because it effectively highlights your leadership abilities, relevant experience, and project success to potential employers.
Functional
Focuses on skills rather than previous jobs
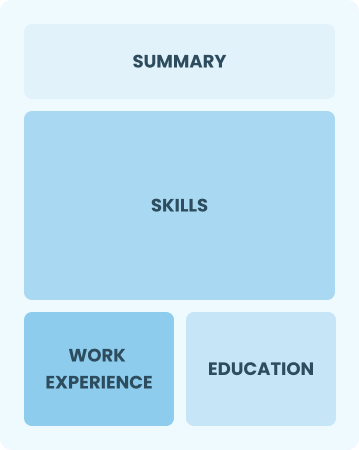
Best for:
Recent graduates and career changers with limited experience in project management
Combination
Balances skills and work history equally
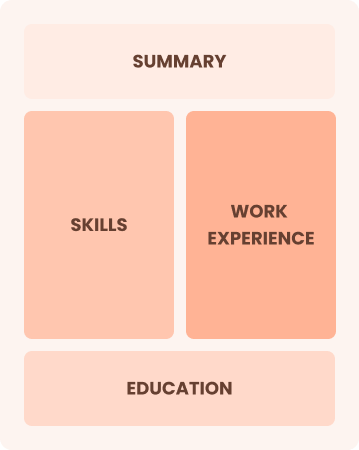
Best for:
Mid-career professionals focused on demonstrating their skills and potential for growth
Chronological
Emphasizes work history in reverse order
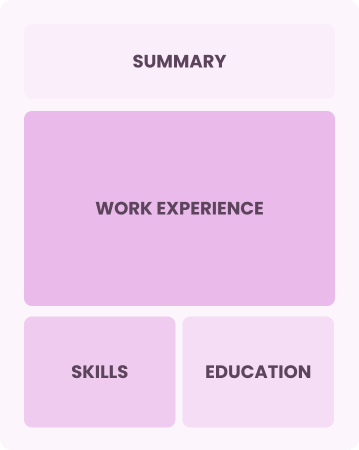
Best for:
Seasoned leaders excelling in complex project management
Frequently Asked Questions
Should I include a cover letter with my project manager resume?
Absolutely, including a cover letter is essential for showcasing your personality and enthusiasm for the position. It allows you to highlight specific experiences that align with the job requirements. If you need help crafting your cover letter, check out our step-by-step guide on how to write a cover letter or use our Cover Letter Generator for quick assistance.
Can I use a resume if I’m applying internationally, or do I need a CV?
When applying for jobs outside the U.S., use a CV instead of a resume to provide a comprehensive overview of your academic and professional history. For guidance on creating an effective CV, explore CV examples that offer templates and tips, or review advice on how to write a CV for proper formatting.
What soft skills are important for project managers?
Soft skills like communication, leadership, and problem-solving are essential for project managers. These interpersonal skills foster collaboration among team members and help navigate challenges effectively, ensuring projects run smoothly and successfully meet their goals.
I’m transitioning from another field. How should I highlight my experience?
Highlight your transferable skills such as communication, teamwork, and project coordination from previous roles. These abilities illustrate your readiness to excel in project management, even if you lack direct experience. Use concrete examples to link your past successes to essential project management tasks like leading teams and meeting deadlines.
Should I use a cover letter template?
Yes, using a cover letter template for project management is advisable as it provides a structured format that organizes your experience in leading teams, managing budgets, and delivering projects on time. This approach effectively highlights relevant skills and achievements, making it easier for hiring managers to recognize your qualifications.
How do I write a resume with no experience?
Even without extensive experience, you can shine as a project manager by highlighting relevant coursework, internships, volunteer projects, and transferable skills like leadership and teamwork. When crafting your resume with no experience, emphasize your adaptability and eagerness to learn. Remember that employers value potential and passion just as much as experience—your unique perspective could set you apart.







