Hard skills are technical abilities such as data analysis, process optimization, and quality assurance testing that ensure products meet industry standards.
Popular Quality Control Resume Examples
Check out our top quality control resume examples that emphasize key skills such as attention to detail, problem-solving, and process improvement. These examples will help you effectively showcase your accomplishments to potential employers.
If you're ready to build your ideal resume, Resume Builder offers user-friendly templates specifically designed for professionals in quality control, making it simple to highlight your strengths.
Recommended
Quality assurance inspector resume
The resume employs a streamlined layout and professional resume fonts that improve readability, enabling hiring managers to quickly grasp key qualifications. These design choices highlight not only the job seeker's accomplishments but also their attention to detail, creating a positive impression on potential employers.
Quality control technician resume
This resume effectively integrates key skills such as quality control processes and statistical process control with relevant work experience. By showcasing these abilities alongside specific accomplishments, employers can clearly understand the applicant's practical expertise and their impact on operational efficiency in manufacturing settings.
Quality analyst resume
This resume skillfully uses bullet points to present accomplishments clearly, allowing hiring managers to quickly grasp the job seeker's impact in quality control roles. The strategic organization of sections and consistent formatting improves readability, ensuring that key skills and achievements are easily identifiable at a glance.
Resume Template—Easy to Copy & Paste
Yuki Lee
Cincinnati, OH 45203
(555)555-5555
Yuki.Lee@example.com
Skills
- Quality Assurance
- Process Improvement
- Data Analysis
- ISO Standards
- Six Sigma
- Root Cause Analysis
- Project Management
- Lean Manufacturing
Certifications
- Certified Quality Engineer - ASQ
- Six Sigma Green Belt - IASSC
Languages
- Spanish - Beginner (A1)
- French - Beginner (A1)
- German - Intermediate (B1)
Professional Summary
Quality Control specialist with 5 years of experience. Proven record in improving product quality by 15%, reducing defect rates by 10%, and enhancing QC protocols. Proficient in ISO standards, Six Sigma, and process improvement.
Work History
Quality Control
Precision Manufacturing Corp - Cincinnati, OH
January 2023 - December 2025
- Ensured product quality improved by 15%
- Implemented audits, reducing defects by 10%
- Developed QC protocols, increasing efficiency
Quality Assurance Specialist
Techtronics Solutions - Cleveland, OH
January 2021 - December 2022
- Monitored QA processes, enhanced by 20%
- Reduced testing time by 30 hours monthly
- Led team in error reduction initiatives
Product Quality Analyst
Innovative Products Inc. - Cleveland, OH
January 2020 - December 2020
- Analyzed defects, cutting costs by 10,000
- Improved defect tracking, up by 25%
- Coordinated actions to exceed quality metrics
Education
Master's Industrial Engineering
University of California Los Angeles, CA
December 2019
Bachelor's Mechanical Engineering
New York University New York, NY
December 2016
How to Write a Quality Control Resume Summary
Your resume summary is the first impression you’ll make on hiring managers, so it’s essential to craft it thoughtfully. As a quality control professional, you should emphasize your attention to detail and analytical skills in this section.
Highlight your ability to identify defects and ensure product excellence, showcasing any relevant certifications or experiences. This will set you apart from other applicants and demonstrate your value.
To illustrate effective strategies for writing a strong summary, we’ll provide some examples that highlight both successful elements and common pitfalls:
I am a quality control professional with several years of experience in the manufacturing industry. I want to find a job where I can apply my skills and help the company achieve its goals. A position that allows for advancement and stability is what I am looking for. I believe I would be a great addition to your team if given the chance.
- Contains vague phrases about experience without detailing specific skills or achievements
- Focuses heavily on personal desires rather than how the job seeker can contribute to the employer's success
- Lacks strong, compelling language that demonstrates confidence and capability in quality control
Results-oriented quality control specialist with over 7 years of experience in manufacturing processes, focusing on product compliance and defect reduction. Achieved a 25% decrease in production defects by implementing rigorous inspection protocols and staff training programs. Proficient in statistical process control, root cause analysis, and ISO standards to ensure the highest quality products.
- Starts with specific years of experience and a clear focus on industry relevance
- Highlights quantifiable achievements that showcase direct impact on product quality
- Mentions relevant technical skills that align with quality control roles, improving credibility
Pro Tip
Showcasing Your Work Experience
The work experience section is a critical component of your resume for quality control positions, as it contains the bulk of your content. Good resume templates always emphasize this important section.
This area should be organized in reverse-chronological order, highlighting your previous roles. Use bullet points to succinctly describe your key achievements and contributions in each position.
To further illustrate effective work history entries for quality control professionals, we’ll provide a couple of examples. These examples will highlight best practices and common pitfalls:
Quality Control Inspector
ABC Manufacturing – Dallas, TX
- Checked products for defects
- Maintained quality standards
- Documented inspection results
- Worked with production teams
- No details about the employment dates
- Bullet points lack specifics on achievements or metrics
- Emphasizes routine tasks instead of effective contributions
Quality Control Specialist
Tech Innovations Inc. – Austin, TX
March 2020 - Present
- Conduct thorough inspections of production processes to ensure compliance with industry standards, leading to a 30% reduction in defects
- Implement new testing protocols that improved product reliability, contributing to a 15% increase in customer satisfaction ratings
- Train and mentor junior staff on quality assurance practices, fostering a culture of continuous improvement within the team
- Starts each bullet with dynamic action verbs to clearly indicate the applicant's contributions
- Incorporates specific metrics that demonstrate the impact of the applicant’s efforts
- Highlights relevant skills that align with quality control responsibilities
While your resume summary and work experience are key components, don't overlook the importance of other sections. Each area deserves proper attention to help you stand out. For detailed guidance, be sure to check out our comprehensive guide on how to write a resume.
Top Skills to Include on Your Resume
A well-defined skills section on your resume can significantly improve your candidacy. It allows you to showcase your technical skills clearly, making it easier for potential employers to see what you bring to the table.
In quality control, emphasize both technical skills and soft skills. Highlight expertise in statistical process control, familiarity with quality management software like Six Sigma tools, and knowledge of ISO standards, while also showcasing attention to detail, communication, and teamwork abilities that support maintaining high product quality.
Soft skills include attention to detail, effective communication, and problem-solving, which are essential for fostering collaboration and maintaining high-quality outcomes in a team environment.
When selecting skills for your resume, it's important to align them with what employers expect from ideal applicants. Many organizations use automated systems that filter out applicants lacking these essential resume skills.
To effectively tailor your application, review job postings closely for insights on which skills are in demand. This strategy not only helps you catch the attention of recruiters but also increases your chances of passing through applicant tracking systems (ATS).
Pro Tip
10 skills that appear on successful quality control resumes
Improve your resume to attract recruiters by highlighting essential skills sought after in quality control roles. You can see these skills effectively showcased in our resume examples, which will help you land interviews with confidence.
By the way, here are 10 key skills you should consider adding to your resume if they align with your expertise and job expectations:
Attention to detail
Analytical thinking
Problem-solving
Quality assurance methodologies
Statistical analysis
Documentation skill
Team collaboration
Time management
Process improvement techniques
Regulatory compliance knowledge
Based on analysis of 5,000+ quality control professional resumes from 2023-2024
Resume Format Examples
Selecting the appropriate resume format for quality control roles is important as it showcases your relevant skills and experiences, making your professional journey clear and compelling to potential employers.
Functional
Focuses on skills rather than previous jobs
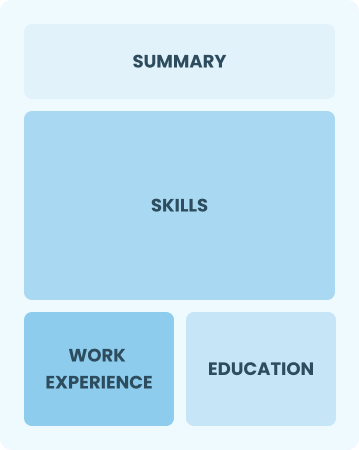
Best for:
Recent graduates and career changers with limited experience in quality control
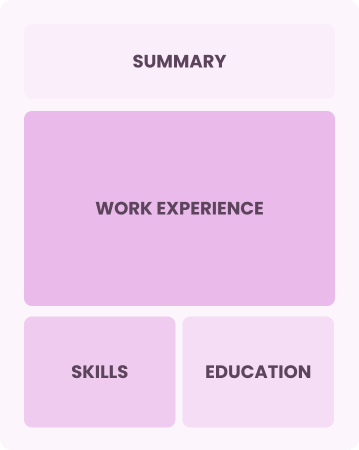
Combination
Balances skills and work history equally
Best for:
Mid-career professionals focused on demonstrating their skills and seeking advancement opportunities
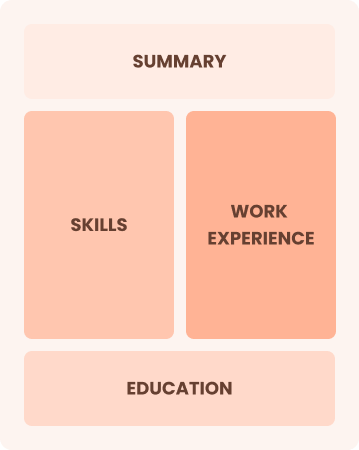
Chronological
Emphasizes work history in reverse order
Best for:
Senior leaders excelling in quality assurance and process improvement
Frequently Asked Questions
Should I include a cover letter with my quality control resume?
Absolutely, including a cover letter is a great way to showcase your qualifications and enthusiasm for the position. It allows you to personalize your application and connect your experience to the job. If you need assistance, explore our resources on how to write a cover letter or use our Cover Letter Generator for quick help.
Can I use a resume if I’m applying internationally, or do I need a CV?
When applying for jobs abroad, use a CV instead of a resume as it is often the preferred format. For guidance on crafting a professional CV that aligns with international expectations, explore our resources on how to write a CV and review various CV examples designed to help you create a compelling document.
What soft skills are important for quality controls?
Soft skills like attention to detail, communication, and problem-solving are essential in quality control. These interpersonal skills foster collaboration among team members and ensure that processes run smoothly, ultimately leading to higher quality products and satisfied clients.
I’m transitioning from another field. How should I highlight my experience?
Highlight your transferable skills such as attention to detail, analytical thinking, and effective communication when applying for quality control positions. Even if your experience is outside the field, these abilities show you can ensure product standards are met. Use specific examples from past roles to illustrate how you've maintained quality in projects or processes.
Where can I find inspiration for writing my cover letter as a quality control?
If you're pursuing quality control positions, explore our expertly crafted cover letter examples. They provide inspiration for your application materials, offering insights on content ideas, formatting tips, and effective ways to showcase your qualifications clearly and professionally.
Should I include a personal mission statement on my quality control resume?
Yes, including a personal mission statement in your resume is advisable. It highlights your values and career aspirations effectively, making it especially compelling for companies that emphasize a strong culture or have missions aligned with your professional goals.







