Hard skills are technical abilities, including food safety regulations, quality control methods, and product formulation techniques that a food technologist needs to master.
Popular Food Technologist Resume Examples
Discover our top food technologist resume examples that emphasize important skills such as product development, quality assurance, and regulatory compliance. These examples demonstrate how to effectively communicate your expertise to potential employers.
Ready to build your ideal resume? Our Resume Builder offers user-friendly templates specifically designed for food industry professionals, helping you make a lasting impression in your job search.
Recommended
Entry-level food technologist resume
This entry-level resume for a food technologist effectively highlights the job seeker's strong background in product development and cost optimization. It showcases achievements from previous roles that reflect their ability to innovate within the food industry. New professionals should demonstrate their technical skills and relevant experiences through measurable accomplishments and certifications, even with limited work history.
Mid-career food technologist resume
This resume effectively showcases qualifications by highlighting a progressive career in food technology. The job seeker’s accomplishments and leadership experiences signal readiness for advanced roles, demonstrating expertise in product development and quality assurance alongside a commitment to innovation.
Experienced food technologist resume
This resume demonstrates the applicant's robust experience as a food technologist, featuring significant achievements like a 15% increase in product efficiency and the development of five new food safety protocols. The clear formatting allows hiring managers to quickly appreciate their effective contributions to the industry.
Resume Template—Easy to Copy & Paste
Larry Garcia
Westbrook, ME 04093
(555)555-5555
Larry.Garcia@example.com
Professional Summary
Dynamic Food Technologist with 9 years of experience in product development, quality assurance, and safety technology. Expert in process optimization and regulatory compliance. Proven track record in enhancing sales and efficiency.
Work History
Food Technologist
GrainMinds Innovations - Westbrook, ME
April 2021 - October 2025
- Developed 15 new product lines boosting sales by 12%
- Improved processing efficiency reducing waste by 8%
- Conducted 25+ quality assurance tests monthly
Food Science Specialist
NutrientBio Corp - Portland, ME
April 2016 - April 2021
- Led team to achieve 95% customer satisfaction ratings
- Reduced formula costs by 15% via ingredient innovation
- Conducted bi-weekly audits to ensure safety standards
Food Quality Analyst
TasteMaster Foods - Portland, ME
April 2014 - March 2016
- Streamlined testing procedures saving 20% in time
- Ensured compliance with all FDA regulations
- Supported cross-department projects improving outputs
Skills
- Product Development
- Quality Assurance
- Nutritional Analysis
- Food Safety Standards
- Regulatory Compliance
- Process Optimization
- Project Management
- Data Analysis
Education
Master of Science Food Science and Technology
University of Illinois Champaign, Illinois
May 2014
Bachelor of Science Food Science
Purdue University West Lafayette, Indiana
May 2012
Certifications
- Certified Food Scientist - Institute of Food Technologists
- HACCP Manager - National Registry of Food Safety Professionals
Languages
- Spanish - Beginner (A1)
- French - Intermediate (B1)
- German - Beginner (A1)
How to Write a Food Technologist Resume Summary
Your resume summary is the first impression employers have of you, making it essential to craft a compelling introduction that showcases your qualifications. As a food technologist, it's important to highlight your knowledge in food safety, product development, and quality assurance.
This profession should emphasize your technical skills, creativity in food innovation, and understanding of regulatory standards. A well-written summary will set you apart from other applicants and demonstrate your unique value.
To help you understand what makes an effective summary for this role, here are some examples that illustrate both successful and less effective approaches:
I am a dedicated food technologist with years of experience in the industry. I hope to find a position that allows me to use my skills and learn more about food processing. It would be great to work in an environment that values teamwork and offers chances for professional development. I believe I can contribute positively if given the chance.
- Lacks specific details about relevant skills and accomplishments in food technology
- Overuses personal pronouns and vague terms, which dilute impact
- Emphasizes what the job seeker seeks from a job instead of highlighting their potential contributions to an employer
Results-driven food technologist with over 6 years of experience in product development and quality assurance within the food industry. Improved product shelf life by 20% through innovative packaging solutions and improved formulation methods. Proficient in food safety regulations, sensory evaluation techniques, and cross-functional team leadership to ensure compliance and deliver high-quality products.
- Begins with a clear indication of the applicant's experience level and area of expertise
- Highlights measurable achievements that showcase the applicant's impact on product quality and innovation
- Lists relevant technical skills that are essential for success in the food technology field
Pro Tip
Showcasing Your Work Experience
The work experience section is important for your resume as a food technologist, serving as the main focus and containing the bulk of your content. Good resume templates always include this essential section to highlight relevant professional experiences.
Organized in reverse-chronological order, this section should list your previous roles along with three to four bullet points detailing your achievements in each position. This format allows hiring managers to quickly grasp your contributions and expertise.
To illustrate effective entries, we’ll present a couple of examples that showcase what works well and what doesn’t in the context of food technology:
Food Technologist
Global Foods Inc. – Los Angeles, CA
- Tested food samples for quality.
- Assisted in product development.
- Maintained lab equipment.
- Conducted research on ingredients.
- Lacks specific details about the projects worked on
- Bullet points do not highlight accomplishments or improvements made
- Focuses on routine tasks instead of demonstrating impact on products or processes
Food Technologist
Fresh Foods Inc. – Chicago, IL
March 2020 - Present
- Develop and optimize food formulations leading to a 30% increase in product shelf life.
- Conduct sensory evaluations that improve customer satisfaction scores by 15% within the first year.
- Collaborate with production teams to streamline processes, reducing waste by 20% and increasing efficiency.
- Uses action verbs at the start of each bullet point to showcase achievements clearly
- Incorporates specific metrics that quantify the impact of the applicant’s contributions
- Highlights relevant skills such as collaboration and innovation, essential for a food technologist
While your resume summary and work experience are important components, don’t overlook the importance of other sections. Each part plays a role in presenting your skills and qualifications effectively. For more detailed guidance, explore our how to write a resume guide.
Top Skills to Include on Your Resume
A well-defined skills section is important for a food technologist's resume. It helps showcase your qualifications and makes it easier for employers to see you possess the essential competencies required for the role.
In this field, highlight technical skills such as skill in food safety regulations, knowledge of quality control systems, and experience with laboratory equipment like spectrophotometers or chromatographs. These details demonstrate your expertise and readiness to contribute effectively.
Soft skills, such as effective communication, teamwork, and problem-solving abilities, play a key role in collaborating with diverse teams and ensuring high-quality food products.
When selecting skills for your resume, it's important to align them with what employers expect in an applicant. Many organizations use automated screening systems that filter out applicants lacking essential resume skills.
To effectively tailor your application, review job postings closely for insights into the specific skills that recruiters seek. This strategy not only improves your chances with human reviewers but also ensures you pass through any applicant tracking systems (ATS) used in the hiring process.
Pro Tip
10 skills that appear on successful food technologist resumes
Highlighting in-demand skills on your resume can significantly attract the attention of recruiters seeking food technologists. These skills are often showcased in resume examples, offering a useful template to craft a strong application.
By the way, incorporating relevant skills that align with your experiences can set you apart from other job seekers. Consider adding any of these key skills that match your qualifications:
Food safety knowledge
Quality control expertise
Product development
Sensory analysis
Regulatory compliance
Nutritional analysis
Research and development skills
Problem-solving abilities
Data analysis skill
Team collaboration
Based on analysis of 5,000+ food service professional resumes from 2023-2024
Resume Format Examples
Choosing the right resume format is essential for a food technologist, as it highlights key skills and experiences that demonstrate your expertise in food safety and product development.
Functional
Focuses on skills rather than previous jobs
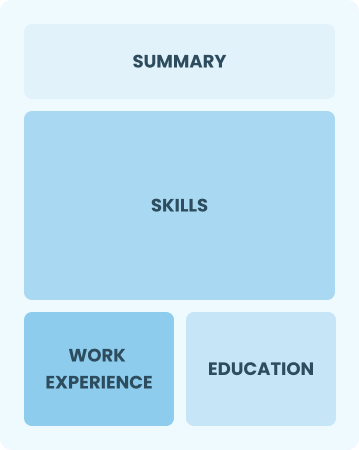
Best for:
Recent graduates and career changers with up to two years of experience
Combination
Balances skills and work history equally
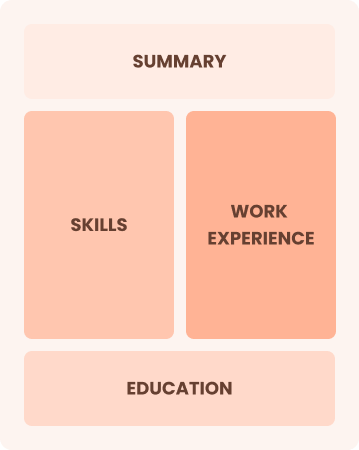
Best for:
Mid-career professionals are focused on demonstrating their skills and growth potential
Chronological
Emphasizes work history in reverse order
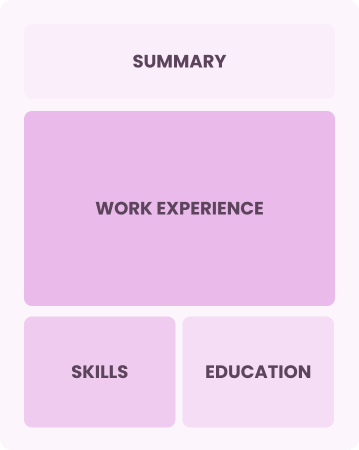
Best for:
Seasoned experts driving innovation in food safety and quality
Frequently Asked Questions
Should I include a cover letter with my food technologist resume?
Including a cover letter is definitely beneficial for making your application more effective, as it allows you to highlight your relevant skills and enthusiasm for the position. If you're seeking assistance, consider our resources on how to write a cover letter. Alternatively, you can use our Cover Letter Generator to streamline the process.
Can I use a resume if I’m applying internationally, or do I need a CV?
When applying for jobs outside the U.S., a CV is often required instead of a resume. A CV provides a comprehensive overview of your career, including education and publications. To help with formatting and writing, explore our detailed resources on how to write a CV tailored for international job seekers. Additionally, you can look at various CV examples to guide you in crafting your own.
What soft skills are important for food technologists?
Soft skills such as communication, teamwork, and problem-solving are essential for food technologists. These interpersonal skills help professionals collaborate effectively with colleagues and engage with consumers, ensuring that food products meet safety standards and consumer needs while fostering a positive work environment.
I’m transitioning from another field. How should I highlight my experience?
Highlight your transferable skills such as teamwork, analytical thinking, and creativity from previous roles. These traits illustrate your potential to thrive in food technology, even if your experience is limited. Provide concrete examples that link your past accomplishments to the responsibilities of a food technologist. This will help you showcase your value effectively.
How should I format a cover letter for a food technologist job?
To format a cover letter, start by including your contact details and a polite greeting. Craft an engaging opening that grabs attention and briefly summarize your qualifications relevant to the food technologist role. Emphasize specific skills or experiences aligning with the job description, and wrap up with a compelling call to action encouraging further discussion.
Should I use a cover letter template?
Yes, using a cover letter template tailored for food technologists is highly advisable because it provides a clear structure to organize content and ensures you effectively highlight key skills such as product development and quality assurance experience that resonate with hiring managers.







