Hard skills are technical abilities, such as project management, systems engineering, and skill in design software, that an engineering manager must excel at to lead projects effectively.
Popular Engineering Manager Resume Examples
Check out our top engineering manager resume examples that showcase leadership skills, project management expertise, and technical skill important for success in this role. These examples will help you highlight your accomplishments effectively.
Ready to build your own powerful resume? Our Resume Builder offers user-friendly templates specifically designed for engineering professionals, making your job application process smoother.
Recommended
Engineering manager resume
The resume uses resume fonts and structured formatting to improve readability, allowing hiring managers to easily assess qualifications. These design choices highlight the applicant's competencies while conveying professionalism and attention to detail, which benefits an early-career engineering manager.
Lead engineer resume
This resume effectively balances skills such as project management and team leadership with extensive engineering experience. By showcasing these abilities alongside a rich work history, employers can clearly see the job seeker's capability to lead projects and drive improvements in complex technical environments.
Engineering team lead resume
This resume effectively uses bullet points to present a wealth of experience clearly and concisely, making it easy for hiring managers to spot key qualifications. The organized layout, including consistent formatting and strategic spacing, highlights significant achievements without clutter, improving overall readability.
Resume Template—Easy to Copy & Paste
Daniel Lee
Indianapolis, IN 46201
(555)555-5555
Daniel.Lee@example.com
Skills
- Leadership
- Project Management
- Software Development
- Agile Methodologies
- Mentoring
- Cost Reduction
- Cross-functional Collaboration
- Performance Optimization
Languages
- Spanish - Beginner (A1)
- French - Beginner (A1)
- Japanese - Beginner (A1)
Professional Summary
Engineering Manager with 8 years of experience leading teams. Expertise in software development, project management, and cross-functional collaboration. Proven track record in enhancing performance and reducing costs.
Work History
Engineering Manager
TechFusion Solutions - Indianapolis, IN
January 2022 - October 2025
- Led team to achieve 20% increase in project efficiency
- Implemented cost-saving measures reducing expenses by 100K annually
- Mentored engineers, resulting in a 15% improvement in performance
Senior Software Engineer
Innovatech Corp - Indianapolis, IN
January 2018 - December 2021
- Developed software that enhanced user engagement by 25%
- Optimized backend systems, reducing server costs by 30%
- Collaborated with cross-functional teams to launch three new products
Software Engineer
Digital Horizons - Greenfield, IN
January 2016 - December 2017
- Implemented databases reducing query times by 50%
- Designed and coded feature upgrades for user interface
- Assisted in training junior developers, enhancing team productivity
Certifications
- Certified ScrumMaster (CSM) - Scrum Alliance
- Project Management Professional (PMP) - Project Management Institute
Education
Master of Science Computer Science
Stanford University Stanford, CA
June 2016
Bachelor of Science Computer Science
University of California, Berkeley Berkeley, CA
June 2014
How to Write a Engineering Manager Resume Summary
Your resume summary is the first thing employers will notice, making it important to create a compelling introduction that captures their attention. As an engineering manager, you should emphasize your leadership abilities and project management skills to stand out in this competitive field.
This profession requires highlighting technical expertise, team collaboration, and successful project delivery. Focus on showcasing your accomplishments and how they align with the potential employer's goals.
To illustrate what makes an effective resume summary for engineering managers, consider the following examples. They will provide insights into crafting a strong opening that resonates with hiring managers:
I am an experienced engineering manager with a solid background in leading teams. I want to find a job where I can use my knowledge and help the company succeed. A supportive work environment with chances for advancement is important to me. I believe I can make a positive impact if given the chance.
- Lacks specific details about the applicant’s accomplishments or skills, making it vague
- Overly focuses on personal desires rather than what value the applicant brings to potential employers
- Uses generic phrases that do not convey confidence or uniqueness, which might fail to capture attention
Results-driven engineering manager with over 8 years of experience in leading cross-functional teams to deliver innovative software solutions. Successfully increased project delivery efficiency by 30% through the implementation of agile methodologies and streamlined communication processes. Proficient in software development lifecycle (SDLC), team leadership, and data analysis, ensuring high-quality outputs that align with business objectives.
- Starts with clear indication of experience level and managerial focus
- Highlights a quantifiable achievement that illustrates impact on project efficiency
- Mentions relevant technical skills and competencies important for an engineering management role
Pro Tip
Showcasing Your Work Experience
The work experience section is important for your resume as an engineering manager and will contain the bulk of your content. Good resume templates are designed to feature this critical section prominently.
This area should be organized in reverse-chronological order, listing your previous positions clearly. Incorporate bullet points to emphasize key achievements and projects that reflect your leadership and technical capabilities.
Now, let’s explore a couple of examples that illustrate effective ways to present your work history. These examples can help clarify what stands out and highlight pitfalls you should avoid:
Engineering Manager
Tech Innovations Inc. – San Francisco, CA
- Managed engineering teams.
- Oversaw project timelines and budgets.
- Collaborated with other departments.
- Conducted meetings and reported progress.
- Lacks specific achievements or results from managing the team
- Bullet points are overly general and do not highlight unique contributions
- No measurable outcomes or impact mentioned regarding projects
Engineering Manager
Tech Innovations Inc. – San Francisco, CA
March 2020 - Present
- Lead a team of 15 engineers in developing cutting-edge software solutions, boosting project delivery speed by 30%.
- Implemented agile methodologies that improved team collaboration and reduced project completion time by 20%.
- Mentor junior engineers, fostering professional growth and ensuring a high-performance culture within the team.
- Uses strong action verbs to clearly communicate the applicant's achievements
- Incorporates specific metrics to demonstrate impact on performance and efficiency
- Highlights relevant skills that align with the role of an engineering manager
While your resume summary and work experience are important components, don’t overlook the significance of other sections. Each part plays a role in showcasing your skills and qualifications. For further insights on crafting an effective resume, be sure to explore our comprehensive guide on how to write a resume.
Top Skills to Include on Your Resume
A skills section is important for showcasing your qualifications and making a strong impression on potential employers. It allows you to highlight technical skills that align with the job description, ensuring your resume stands out.
As an engineering manager, focus on both technical and leadership skills. Highlight tools like CAD software, project management systems such as Jira, and methodologies like Agile to demonstrate your expertise in managing projects effectively.
Soft skills, including leadership, communication, and problem-solving, foster collaboration and drive team success in achieving engineering goals.
When selecting resume skills, it’s important to align them with what employers expect from an ideal applicant. Many organizations employ automated screening systems that can filter out applicants who lack essential qualifications.
To increase your chances of getting noticed, closely review job postings for insights on which skills to emphasize. This approach helps ensure your resume appeals to both ATS filters and recruiters alike.
Pro Tip
10 skills that appear on successful engineering manager resumes
Improve your resume to grab the attention of hiring managers by highlighting key skills that are highly sought after in engineering management. These skills can significantly boost your chances of landing an interview, and you can see many of them highlighted in our resume examples for added inspiration.
Here are 10 essential skills you should consider adding to your resume if they align with your experience and the job requirements:
Leadership
Project management
Technical expertise
Problem-solving
Team collaboration
Budget management
Risk assessment
Quality assurance
Time management
Adaptability
Based on analysis of 5,000+ engineering professional resumes from 2023-2024
Resume Format Examples
Choosing the appropriate resume format is important as it showcases your technical expertise, leadership experience, and career advancements in a way that resonates with potential employers.
Functional
Focuses on skills rather than previous jobs
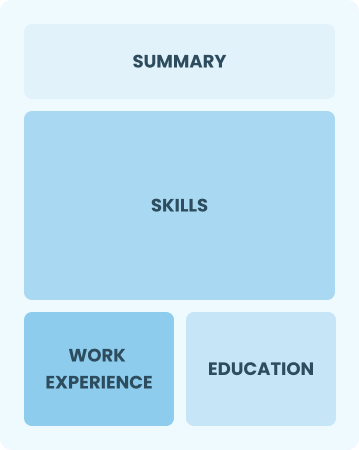
Best for:
Recent graduates and career changers with up to two years of experience
Combination
Balances skills and work history equally
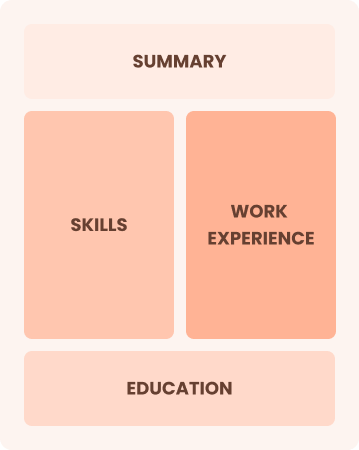
Best for:
Mid-career professionals eager to demonstrate their skills and pursue new opportunities
Chronological
Emphasizes work history in reverse order
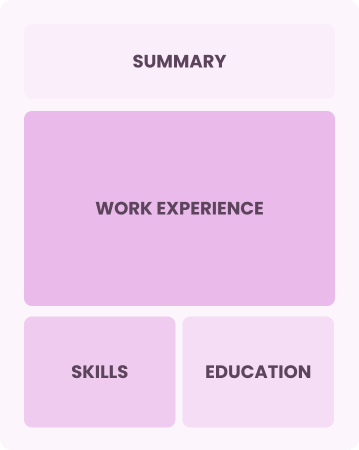
Best for:
Seasoned leaders driving innovative engineering solutions and team growth
Engineering Manager Salaries in the Highest-Paid States
Our engineering manager salary data is based on figures from the U.S. Bureau of Labor Statistics (BLS), the authoritative source for employment trends and wage information nationwide.
Whether you're entering the workforce or considering a move to a new city or state, this data can help you gauge what fair compensation looks like for engineering managers in your desired area.
Frequently Asked Questions
Should I include a cover letter with my engineering manager resume?
Absolutely. Including a cover letter can significantly improve your application by adding depth to your qualifications and showing your enthusiasm for the position. It’s a chance to highlight relevant experiences that may not be fully captured in your resume. For helpful tips on crafting an effective cover letter, take a look at our comprehensive guide on how to write a cover letter or use our Cover Letter Generator for quick assistance.
Can I use a resume if I’m applying internationally, or do I need a CV?
When applying for jobs abroad, a CV is often required instead of a resume. This is common in Europe and other regions. For guidance, explore CV examples to see properly formatted CVs and learn more about how to write a CV tailored to international standards.
What soft skills are important for engineering managers?
Soft skills like leadership, communication, and interpersonal skills are essential for engineering managers. These skills foster collaboration among team members and ensure effective project execution, ultimately driving innovation and maintaining a positive workplace culture.
I’m transitioning from another field. How should I highlight my experience?
Highlight your transferable skills like team leadership, strategic planning, and analytical thinking when applying for engineering manager roles. These abilities demonstrate you can navigate challenges effectively, even if your background isn't in engineering. Share concrete examples from past positions that showcase how you’ve driven projects to success and led teams to achieve goals.
How should I format a cover letter for a engineering manager job?
To format a cover letter for engineering manager positions, start with your contact details, followed by a professional greeting. The opening should grab the reader's attention, and the body must clearly outline your relevant experience and skills. Always tailor your content to align with the job requirements. Conclude with a strong closing statement that encourages further discussion.
Should I use a cover letter template?
Yes, using a cover letter template is advisable for an engineering manager, as it offers a clear structure and helps organize your content to effectively showcase leadership skills, project management experience, and technical expertise that resonate with hiring managers.







