Technical abilities like knowledge of access control systems, security protocols, and data management are some examples of hard skills that ensure the protection of sensitive information.
Popular Access Control Specialist Resume Examples
Check out our top access control specialist resume examples that emphasize important skills like security management, risk assessment, and system administration. These resumes will help you effectively showcase your qualifications to potential employers.
Ready to build your ideal resume? Our Resume Builder offers user-friendly templates designed specifically for security professionals, making the process straightforward and efficient.
Recommended
Entry-level access control specialist resume
This entry-level resume highlights the job seeker’s expertise in access control management and significant achievements, such as successfully reducing security breaches by 15%. New professionals in this field must effectively communicate their technical skills and project experiences to demonstrate their readiness for the role, even with limited work history.
Mid-career access control specialist resume
This resume effectively presents qualifications by showcasing strong achievements and technical skills. The clear progression from technician to specialist illustrates the job seeker's readiness for higher responsibilities, emphasizing their proactive approach to security and compliance.
Experienced access control specialist resume
The work history section illustrates the applicant's robust experience as an access control specialist, demonstrating effective leadership in improving security protocols. Key achievements include a 30% increase in protocol efficiency and a remarkable 50% reduction in incident response time, with bullet points improving clarity for hiring managers.
Resume Template—Easy to Copy & Paste
Sophia Singh
Spokane, WA 99206
(555)555-5555
Sophia.Singh@example.com
Professional Summary
Experienced Access Control Specialist adept in implementing superior security systems, enhancing compliance, and reducing incidents with proven expertise in risk management and staff training. Achieved significant improvements in security efficiency and breach reduction.
Work History
Access Control Specialist
SecureTech Solutions - Spokane, WA
May 2023 - September 2025
- Managed access for 500+ employees, reducing incidents by 30%
- Implemented new protocols boosting security efficiency by 25%
- Coordinated security audits resulting in 100% compliance
Security Systems Advisor
EagleEye Security - Silverlake, WA
January 2018 - April 2023
- Supervised team ensuring 24/7 system operability
- Optimized security systems, reducing breaches by 40%
- Trained staff on security measures, improving response time by 35%
Access Management Coordinator
SafeGuard Innovations - Tacoma, WA
January 2016 - December 2017
- Developed access control policies, enhancing compliance by 20%
- Maintained security databases for 2000+ users
- Conducted audits, reducing unauthorized access by 15%
Skills
- Access Control Systems
- Security Protocol Implementation
- Risk Management
- Incident Response
- Security Audits
- Database Management
- Staff Training
- Compliance Management
Education
Master's Degree Information Security
University of Washington Seattle, Washington
June 2015
Bachelor's Degree Computer Science
Oregon State University Corvallis, Oregon
June 2013
Certifications
- Certified Information Systems Security Professional - ISC2
- Certified Access Control Specialist - ASIS International
- Cybersecurity Certification - CompTIA
Languages
- Spanish - Beginner (A1)
- French - Intermediate (B1)
- German - Intermediate (B1)
How to Write an Access Control Specialist Resume Summary
Your resume summary is the first opportunity to make an impression on hiring managers, so it's essential to present your qualifications clearly and confidently. As an access control specialist, you should emphasize your expertise in security protocols, risk assessments, and system implementation.
Highlighting your technical skills and experience with access control systems will set you apart from other applicants. This role demands attention to detail and a proactive approach to ensuring safety and compliance.
To illustrate what works in this key section, review the following examples that demonstrate effective resume summaries:
I am an access control specialist with many years of experience in security systems. I hope to find a job that allows me to use my skills and make the workplace safer. A position where I can be part of a great team is what I am looking for, as it would help me grow professionally.
- Lacks specific information about the applicant’s expertise in access control technologies or relevant certifications
- Relies on personal desires rather than detailing how the applicant can improve organizational security
- Uses generic phrases like "many years of experience" without quantifying achievements or contributions
Detail-oriented access control specialist with 7+ years of experience in managing security systems and protocols for corporate environments. Improved system efficiency by 20% through the implementation of advanced biometric authentication technologies, resulting in improved facility safety and reduced unauthorized access incidents by 30%. Proficient in surveillance systems, identity verification processes, and compliance regulations.
- Begins with specific years of experience and expertise in security management
- Highlights quantifiable achievements that illustrate a significant impact on facility safety
- Showcases relevant technical skills and knowledge that align with industry standards
Pro Tip
Showcasing Your Work Experience
The work experience section is important for your resume as an access control specialist, where you'll present the majority of your qualifications. Good resume templates always emphasize this important area.
This section should be organized in reverse-chronological order, detailing your previous roles. Use bullet points to highlight key achievements and responsibilities that demonstrate your expertise in access control.
To assist you further, we’ll share examples that illustrate effective work history entries for access control specialists. These examples will show you what stands out and what aspects to avoid:
Access Control Specialist
SecureTech Solutions – Dallas, TX
- Monitored access points.
- Checked IDs and verified credentials.
- Helped enforce security protocols.
- Reported incidents to management.
- Lacks specific employment dates
- Doesn't highlight any measurable achievements or impact
- Describes basic responsibilities rather than demonstrating effectiveness in the role
Access Control Specialist
SecureTech Solutions – San Francisco, CA
March 2020 - Current
- Implement security protocols that reduced unauthorized access incidents by 40% over the past year.
- Conduct regular audits and assessments of access systems, improving compliance with industry standards.
- Train new staff on access control procedures, ensuring a seamless onboarding process and adherence to safety regulations.
- Uses strong action verbs to illustrate significant contributions made in the role
- Incorporates specific metrics to demonstrate effectiveness and impact of responsibilities
- Highlights relevant skills and best practices essential for success in access control management
While your resume summary and work experience are important components, don't overlook the significance of other sections. Each part plays a key role in showcasing your qualifications. For more detailed guidance, explore our comprehensive guide on how to write a resume.
Top Skills to Include on Your Resume
A well-defined skills section is important for making your resume stand out. It allows you to quickly demonstrate to potential employers that you possess the necessary qualifications for the access control specialist role.
Your resume should include a mix of hard and soft skills. Including a diverse range of relevant skills will make your application much stronger.
Skills such as problem-solving, attention to detail, and effective communication are considered soft skills, which are important for coordinating with team members and safeguarding organizational assets.
Selecting the right resume skills is important to align with what employers expect from applicants. Many companies use automated screening systems that filter out applicants lacking these essential qualifications.
To effectively showcase your skills, carefully examine job postings for insights on which attributes are most valued by recruiters and ATS systems alike. This approach will help you emphasize the key competencies that can improve your visibility and appeal as a job seeker.
Pro Tip
10 skills that appear on successful access control specialist resumes
Elevate your resume by highlighting essential skills that are highly sought after in access control specialist roles. You can find these skills illustrated in various resume examples, giving you the assurance needed to make a strong application.
By the way, consider integrating these key competencies into your resume if they align with your qualifications and job specifications:
Attention to detail
Problem-solving
Technical skill
Knowledge of security protocols
Communication skills
Analytical thinking
Risk assessment
Customer service orientation
Surveillance systems expertise
Incident response planning
Based on analysis of 5,000+ administrative professional resumes from 2023-2024
Resume Format Examples
Selecting the appropriate resume format is important for an access control specialist, as it effectively emphasizes relevant security skills, experience, and career growth to potential employers.
Functional
Focuses on skills rather than previous jobs
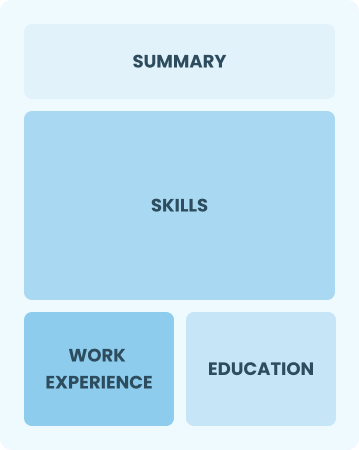
Best for:
Recent graduates and career changers with up to two years of experience
Combination
Balances skills and work history equally
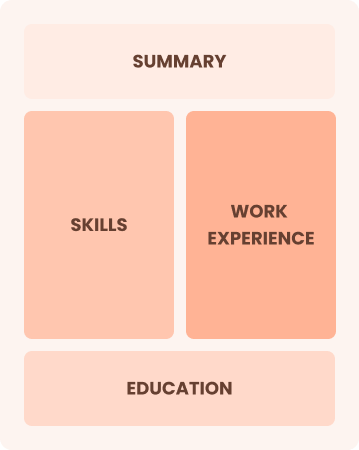
Best for:
Mid-career professionals focused on demonstrating skills and growth potential
Chronological
Emphasizes work history in reverse order
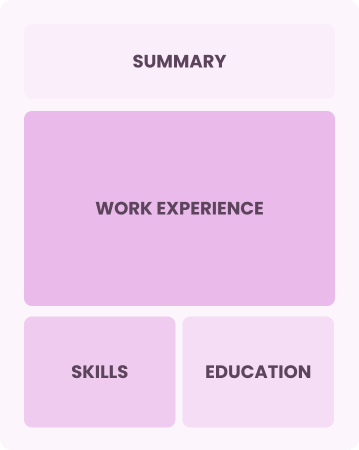
Best for:
Seasoned experts in strategic security and compliance leadership
Frequently Asked Questions
Should I include a cover letter with my access control specialist resume?
Absolutely, including a cover letter can significantly improve your application by allowing you to showcase your personality and relevant skills. It provides an opportunity to explain why you're the perfect fit for the position. For those looking for assistance, consider checking out our comprehensive guide on how to write a cover letter or use our Cover Letter Generator for a quick start.
Can I use a resume if I’m applying internationally, or do I need a CV?
When applying for jobs abroad, use a CV instead of a resume as it's often the preferred format in many regions. To assist you, we offer comprehensive resources that illustrate how to write a CV and creation techniques tailored to international standards. Additionally, explore CV examples to see proper formatting in action.
What soft skills are important for access control specialists?
Soft skills like attention to detail, communication, and problem-solving are essential for access control specialists. These interpersonal skills help ensure effective collaboration with teams and foster trust with clients, ultimately improving security protocols and promoting a safe environment.
I’m transitioning from another field. How should I highlight my experience?
Highlight your transferable skills, such as analytical thinking, attention to detail, and teamwork from previous roles. These abilities are important for an access control specialist and show your potential to excel despite limited direct experience. Use concrete examples to illustrate how your past achievements relate to security protocols and risk management responsibilities.
Where can I find inspiration for writing my cover letter as a access control specialist?
Job seekers in access control should explore our expertly crafted cover letter examples, which provide valuable content ideas, formatting tips, and insights on showcasing your qualifications effectively. Use these samples as a guide to create compelling application materials that stand out in this competitive field.
How do I write a resume with no experience?
If you're writing a resume with no experience, highlight relevant skills like attention to detail, problem-solving, and technological skill. Mention any training, certificates, or projects you've completed that relate to security systems. Your eagerness to learn and adapt will certainly catch the eye of employers.







